Silicon has long served as the essential semiconductor in photovoltaic panels. Yet a new study led by the Institute for Computational Science and Artificial Intelligence at Van Lang University, in the capital of Viet Nam shows various compounds have better bands gaps to improve on the current perovskite materials in wide use.
This figure shows the Red atoms, Green atoms and Blue atoms (which would be at the centre of each octahedron), in a Ruddlesden-Popper structure in the ratio 4:2:1. The paper pulls together research of various combinations of elements in these positions.
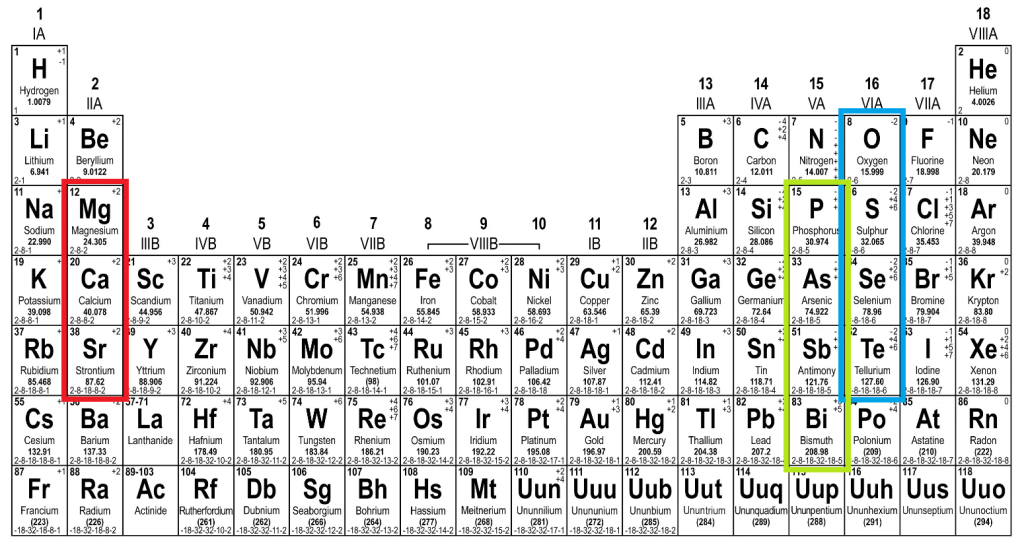
- For red, group 2 elements: Magnesium, calcium and strontium (Sr)
- For green, nitrogen-like group 5 elements: Bismuth (Bi), antimony (Sb), arsenic, phosphorus
- For blue, group 6 elements: Tellerium, selenium, sulphur and our old friend, oxygen.
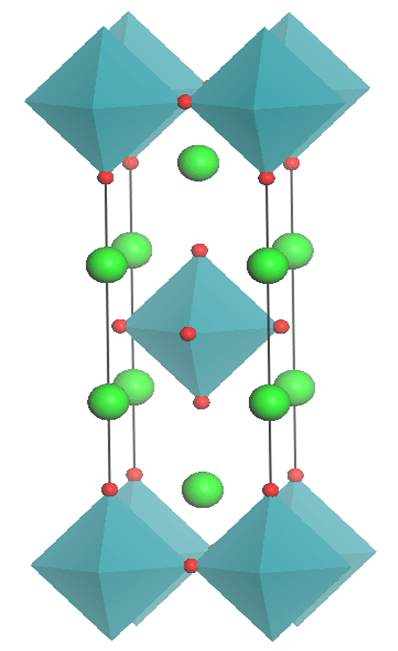
Sr4Sb2O and Sr4Bi2O were found to be the most promising compounds for more efficiently turning light to electricity.
Source
Promising photovoltaic, optoelectronic and p-type thermoelectric Sr4Pn2O (Pn = Sb, Bi) compounds: A first principles study, Chemical Physics, 2024-06-29
